Navigating Post-Market Surveillance for Medical Devices in the EU
The landscape of medical device regulation in the European Union (EU) has undergone significant changes with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). One critical component of these regulations is post-market surveillance (PMS), a systematic process to monitor the safety and performance of medical devices after they have been placed on the market. Understanding and complying with PMS requirements is essential for manufacturers to ensure ongoing compliance and patient safety.
What is Post-Market Surveillance?
Post-market surveillance involves activities carried out by manufacturers to continuously assess the quality, performance (benefits), and safety (risks) of their medical devices once they are placed on the market. This systemic and proactive approach aims to identify and mitigate potential risks and to gather clinical data on how the device is used. The goal is to ensure that the device is performing as intended and that any potential issues are promptly addressed, so that the benefit-risk ratio continues to be acceptable.
Why is Post-Market Surveillance Important?
- Patient Safety: Continuous monitoring helps to detect adverse events and potential hazards, protecting patients from harm.
- Regulatory Compliance: Adhering to PMS requirements is mandatory under the MDR, ensuring that manufacturers stay compliant with EU regulations.
- Product Improvement: Data collected through PMS can provide insights into the device performance, leading to improvements and innovations.
- Market Trust: Demonstrating commitment to safety and quality enhances the reputation and trustworthiness of manufacturers in the market.
Key Components of Post-Market Surveillance
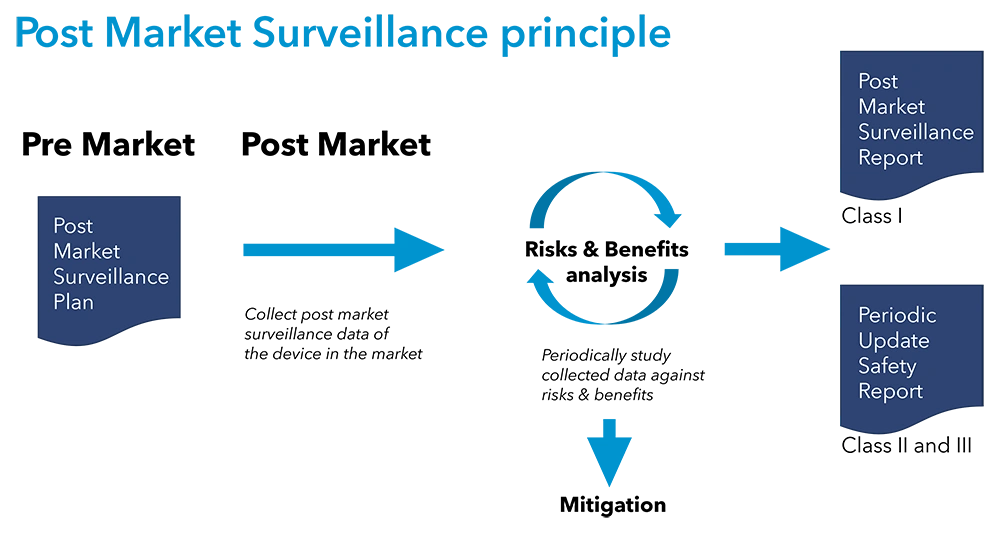
- PMS Plan: Every manufacturer must develop a PMS plan outlining the systematic process for gathering and evaluating information on device performance and safety. The plan should detail the methods for data collection, analysis, and reporting.
- Periodic Safety Update Report (PSUR) or PMS Report (PMSR): Manufacturers of class IIa, IIb, and III devices must prepare a PSUR, and manufacturers of Class I devices must prepare a PMSR, summarizing the results and conclusions of the PMS data. These reports are updated at regular intervals and reviewed by notified bodies.
- Post-Market Clinical Follow-up (PMCF): PMCF is an essential part of PMS, focusing on gathering clinical data to confirm the safety and performance of the device. It involves conducting clinical investigations or observational studies in the post-market phase.
- Vigilance System: This involves reporting and evaluating incidents and Field Safety Corrective Actions (FSCAs). Manufacturers must have a system in place to report serious incidents to the relevant Competent Authorities within specified timeframes.
Implementing an Effective PMS System
- Data Collection: Utilize various sources such as user feedback, complaints, manufacturing quality information, literature reviews, and registries to gather comprehensive data on device performance.
- Clinical Evaluation and Risk Management: Integrate PMS findings into the clinical evaluation and risk management processes, updating benefit-risk assessments and mitigation measures as necessary. Partnering with an ISO 13485-certified organization like BAAT ensures these processes are both compliant and efficient.
- Training and Resources: Ensure that staff involved in PMS activities are adequately trained and that sufficient resources are allocated to maintain an effective PMS system.
- Stakeholder Communication: Maintain open lines of communication with all stakeholders including e.g. distributors, healthcare providers, patients, and regulatory bodies. Timely dissemination of relevant information is crucial for effective surveillance.
Challenges and Best Practices
Challenges
- Data Volume and Quality: Handling large volumes of data from various sources and ensuring its accuracy can be daunting.
- Regulatory Changes: Keeping up with evolving regulations and standards requires continuous effort and vigilance.
- Resource Allocation: Implementing and maintaining a robust PMS system demands significant resources, both in terms of personnel and finances.
Best Practices
Proactive Approach: Adopt a proactive rather than reactive approach to PMS, focusing on early detection and prevention of issues.
Continuous Improvement: Regularly review and update PMS processes to align with the latest regulatory requirements and industry best practices.
Collaboration: Engage with stakeholders, including healthcare professionals and patients, to gather valuable insights and feedback.
Experience matters
Post-market surveillance is a critical aspect of the lifecycle of medical devices in the EU, ensuring that devices remain safe and effective throughout their use. By developing and maintaining a comprehensive PMS system, you can not only comply with regulatory requirements but also enhance patient safety and drive continuous improvement in your products. As the regulatory landscape evolves, staying informed and proactive in PMS activities will be key to navigating the complexities of the medical device market in the EU.
BAAT Medical can help you to understand, navigate, develop and maintain a comprehensive and complying PMS system. This will smoothen the various roles and stakeholders and their needs and assists in generating the required documentation for you.




