Medical Device Development
Expertise at your fingertips
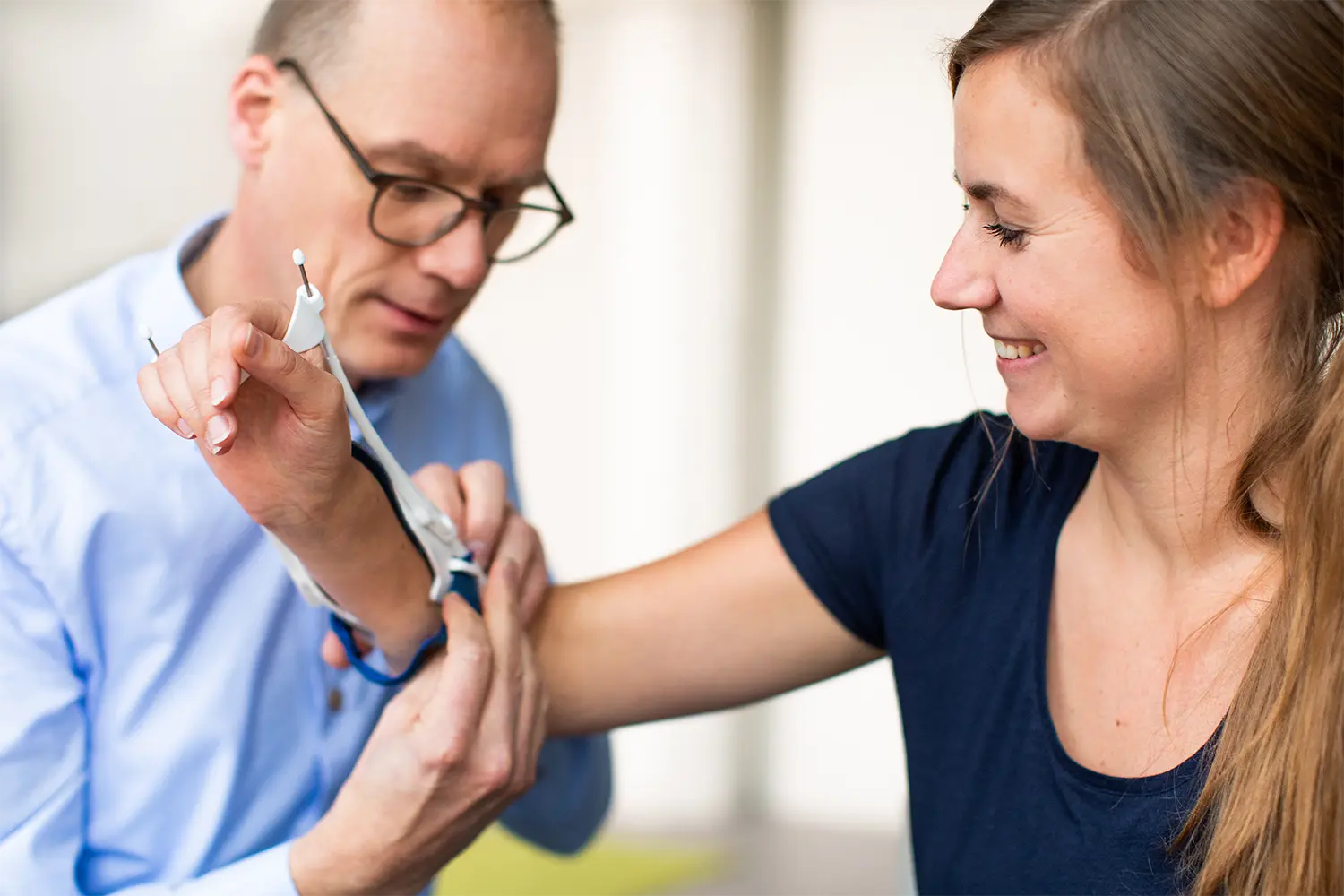
Do you have a great idea and need a partner for the design and manufacture of medical devices? Is your development portfolio bigger than your R&D department’s capacity? BAAT is your partner in expert medical device development from prototype to regulatory approval. BAAT has the experience and know-how to transform your idea into a medical device with manufacturing route, CE/FDA application and operations.
Design control medical device?
Medical device development is the process used to transform your idea into an approved medical device that is treating patients. BAAT has been the go-to company for medical device development for many customers for over two decades. For us, it is not just about industrial design sketches, medical device prototyping; it is about creating a meaningful impact on the lives of patients.
Along the journey from idea to regulatory approval, you will encounter hurdles. BAAT is successfully guiding customers to overcome these hurdles since 1999. Use this experience to navigate the intricacies of your medical device design and development and get your medical device approved for market introduction.
Medical device product development
Even the best medical device developers are only as good as the medical device development process they are working under. At least according to the authorities assessing market approval. Strict design controls for the medical device industry are in place, meaning an expert team is not enough to get regulatory approval for a medical device.
They must use a formal medical device development process, with activities focused on design control. The goal of the design control process is the reliable design of medical devices, resulting in products that are safe and effective. For design control, medical device development includes the following topics:
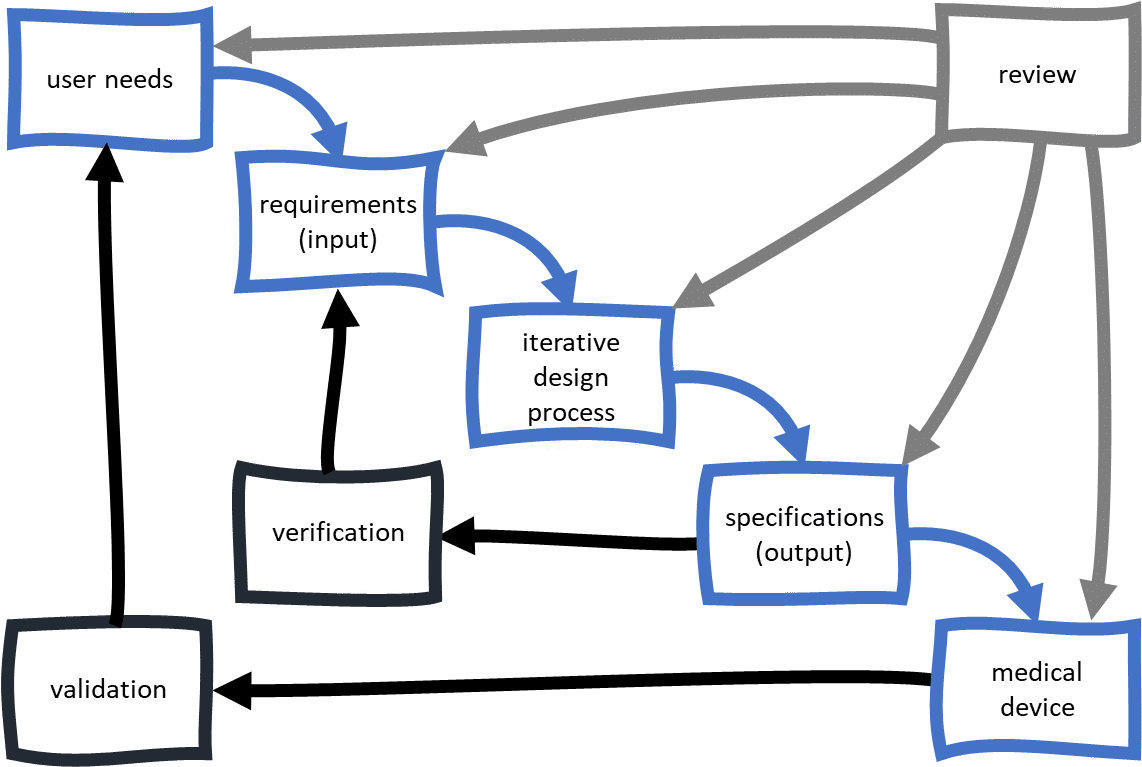
User needs
- What do users want to achieve with the medical device?
What is the clinical benefit?
In what environment does it need to work?
Requirements (input)
- What must the medical device be capable of? What qualities must it have?
Iterative design process
- How can the device fulfil the user needs and requirements?
Specification (output)
- What attributes and characteristics describe the device, packaging and information that comes with it?
Verification
- How can we prove the specified design (output) meets the requirements (input)? Or, according to the FDA: ‘Did we design the device right?
Validation
- How can we ensure that the device fulfils the user needs? Or, according to the FDA: ‘Did we design the right device?’