Do you have a great idea for a medical device and need a partner to realize it and make it ready for market approval? Is your development portfolio bigger than your R&D department’s capacity?
BAAT is your partner in expert medical device design engineering. BAAT has the experience and know-how to transform your dream, idea, technology or problem into a product with manufacturing route, CE/FDA application and operations.
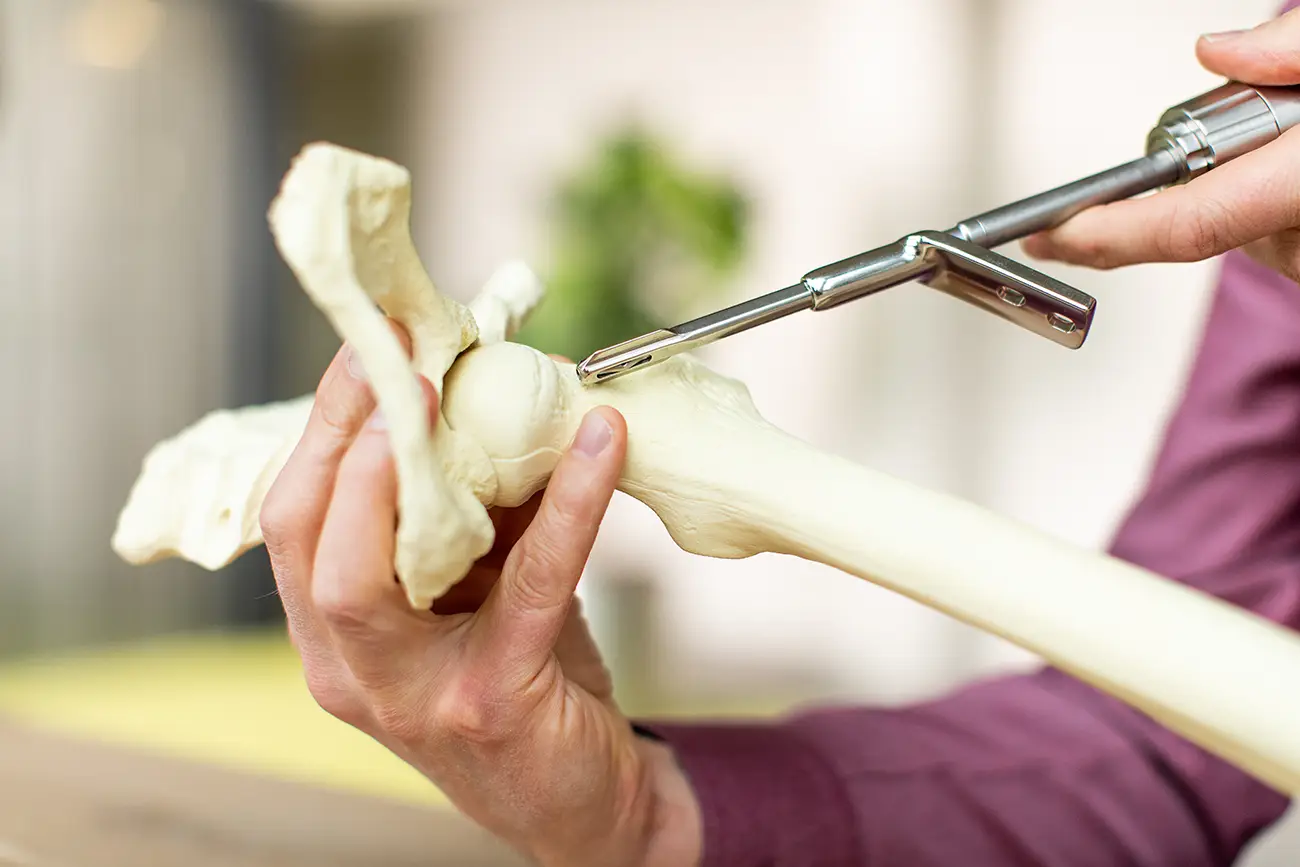
Medical device engineering is applying engineering principles for healthcare purposes. It is not just calculations, drawings and tolerances; it is about creating a meaningful impact on the lives of patients. During the journey from idea to product there are important questions to answer, many of them not of technical nature:
Asking the right questions is the first step to a successful project. Having impact on the end user means impact in the market. Some examples:
One of the steps many customers do is answering above questions using an Innovation Scan. We call it ‘Innoscan’. Get in contact with Baat to see what can lead to a commercially viable product development and real impact in the medical device business.

Our ISO 13485 quality system (certificates) concerns topics directly related to product development (e.g. verification and validation, risk management, usability), but also addresses many high-level topics such as competence tracking and training, management of standards, control of documents.
When partnering with BAAT, you will not only benefit from the knowledge and experience of our 25+ people with various fields of expertise, our expertise with medical device engineering for both the EU and US markets with 30+ products developed (publication list), but also of our underlying quality system.
Even the best and most experienced medical device design engineers are only as good as the quality system they are working under. At least according to the authorities assessing market approval. That means a team of experts in medical product engineering is not enough; they must use a thorough quality management system to get regulatory approval for a medical device.

Business development
"*" indicates required fields