As a medical device manufacturer and as a medical device startup, your responsibilities don’t end when your orthopedic or other medical device product innovation launches on the market. In the EU for example, you must keep an eye on the product for as long as it is on the market according to various (MDR) regulations.
You have a great innovation for a medical device. Now, what are the first steps to take apart from getting investors on board and raise funding? Think about realising post market surveillance (PMCF) from the project start and the feasibility of commercial succes greatly improves. BAAT has the experience and know-how to manage and service all requirements that medical startups encounter. This includes the manufacturing route, CE/FDA application but PMCF as well. You focus on marketing and sales (and getting investors on board) and let us take away the pains that come with ensuring traceability of medical device innovations. Get ready for launch without headaches and hurdles that will come your way. Read all about do’s and don’ts concerning (post) market surveillance and feel free to contact us to get more info on how to move your project forward sustainably.
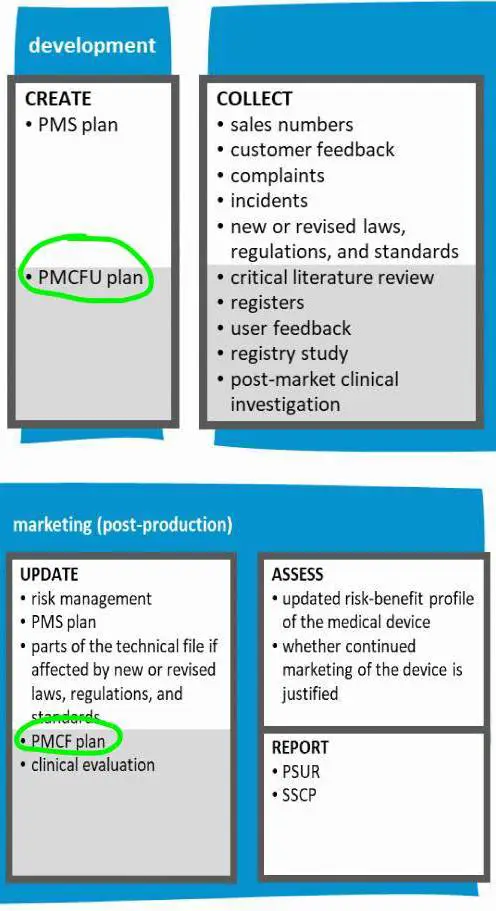
When a medical device enters the market, the post market surveillance or pmfc plan comes into action. Usually, this includes regular assessment of sales numbers, customer feedback, complaints, incidents and tracking of new/revised laws, regulations and standards. Risk management, Clinical evaluation, technical documentation and the PMS plan are updated with this new information to keep the technical file state of art. In addition, a post market clinical follow up evaluates the safety and performance of the device. Based on this, the manufacturer regularly confirms that the risk-benefit profile of the medical device is favourable and the product can continue to improve the lives of patients.

Post market clinical follow up is part of the post market surveillance process, specifically the part that keeps the clinical safety and performance profile of a medical device up to date. Like the post market surveillance, it already starts with a post market surveillance plan (PMCF plan) in the development phase. The goals of a medical device PMCF are to:
The post market clinical follow up plan describes when, where and how to systematically gather information to address these topics.
Once the medical device is being used, the activities in the plan are executed. Our clinical research experts dive into registers, perform critical literature reviews, and assess user feedback. Depending on the risks and unknowns of the device, the PMCF can include post market registry studies and/or post-market clinical investigations with the medical device.
The results are used in the post market surveillance to assess the risk-benefit profile of the medical device and confirm that you can continue selling it. Risk management, Clinical evaluation, technical documentation and the PMS plan are updated with this new information to keep the technical file state of art.

The results and conclusion of the post market surveillance are written down in a periodic safety update report (PSUR); this report is created periodically for the lifetime of the device. Depending on the classification of the device there is a requirement to add this to the technical documentation regularly, at least annually, or even to submit it at the notified body for review each year.

For implantable devices and class III devices, information about the safety and performance must be available to everybody. As legal manufacturer you must create a summary of safety and clinical performance (SSCP) and submit it to the notified body. The notified body reviews and uploads it in a publicly accessible database (Eudamed).
Discover your own potential and focus on entrepreneurship by outsourcing medical product engineering versus investing in organizing all activities, responsibilities and a quality management system in house.

Business development
"*" indicates required fields