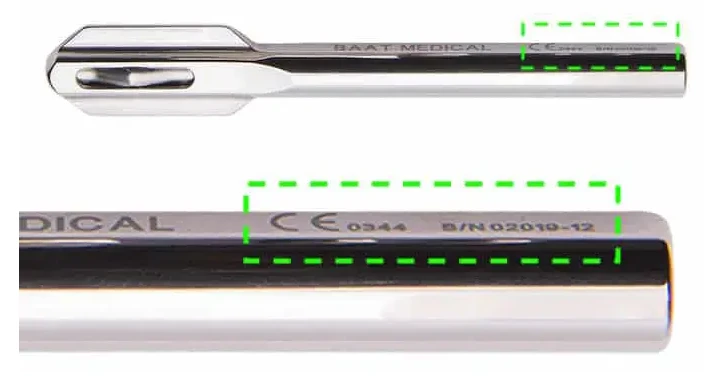
What is CE Marking Required for Medical Devices?
Understanding the significance of CE marking for medical devices is crucial when marketing these products within the European Union (EU). CE marking certifies compliance with rigorous health and safety requirements mandated by EU legislation. In this article, we explore why CE marking is necessary for medical devices and the key steps to obtain this certification.
The Importance of CE Marking for Medical Devices
CE marking is mandatory for medical devices in the EU, indicating adherence to essential safety, performance, and quality standards. It holds significant importance:
- Ensuring Compliance: CE marking confirms compliance with EU regulations, assuring manufacturers and users of the device’s adherence to safety standards.
- Market Access: CE marking allows free market access and distribution within the EU, facilitating growth and expansion for manufacturers.
- Enhancing Trust: CE marking instills confidence in healthcare professionals, patients, and regulatory authorities, promoting device adoption.
The Process of Achieving CE Marking
Obtaining CE marking involves several steps to ensure regulatory compliance:
- Device Classification: Medical devices are classified based on risk level, determining conformity assessment and notified body involvement.
- Conformity Assessment: Lower-risk devices are self-assessed by manufacturers, while higher-risk devices require notified body involvement.
- Notified Body Evaluation: Manufacturers select an appropriate notified body to evaluate technical documentation, quality management, and manufacturing processes.
- Technical Documentation: Manufacturers prepare comprehensive documentation showcasing device conformity and compliance.
- Clinical Evaluation: Thorough evaluation of clinical data demonstrates device safety and performance.
- Declaration of Conformity: The manufacturer issues a declaration certifying compliance with EU regulations.
- Affixing the CE Mark: The CE mark is affixed to the device, packaging, and documentation, symbolizing conformity.
The Role of Notified Bodies
Notified bodies play a crucial role in the CE marking process:
- Independent Assessment: Notified bodies evaluate device conformity, ensuring safety and performance standards are met.
- Choice and Collaboration: Manufacturers choose notified bodies that align with their requirements, streamlining the certification process.
Conclusion
CE marking is vital for medical devices in the EU, signifying compliance with stringent standards. Understanding the significance of CE marking, following the prescribed steps, and collaborating with notified bodies ensure successful certification and market access. By adhering to these processes, manufacturers confidently introduce innovative and safe medical devices to benefit healthcare professionals and patients.