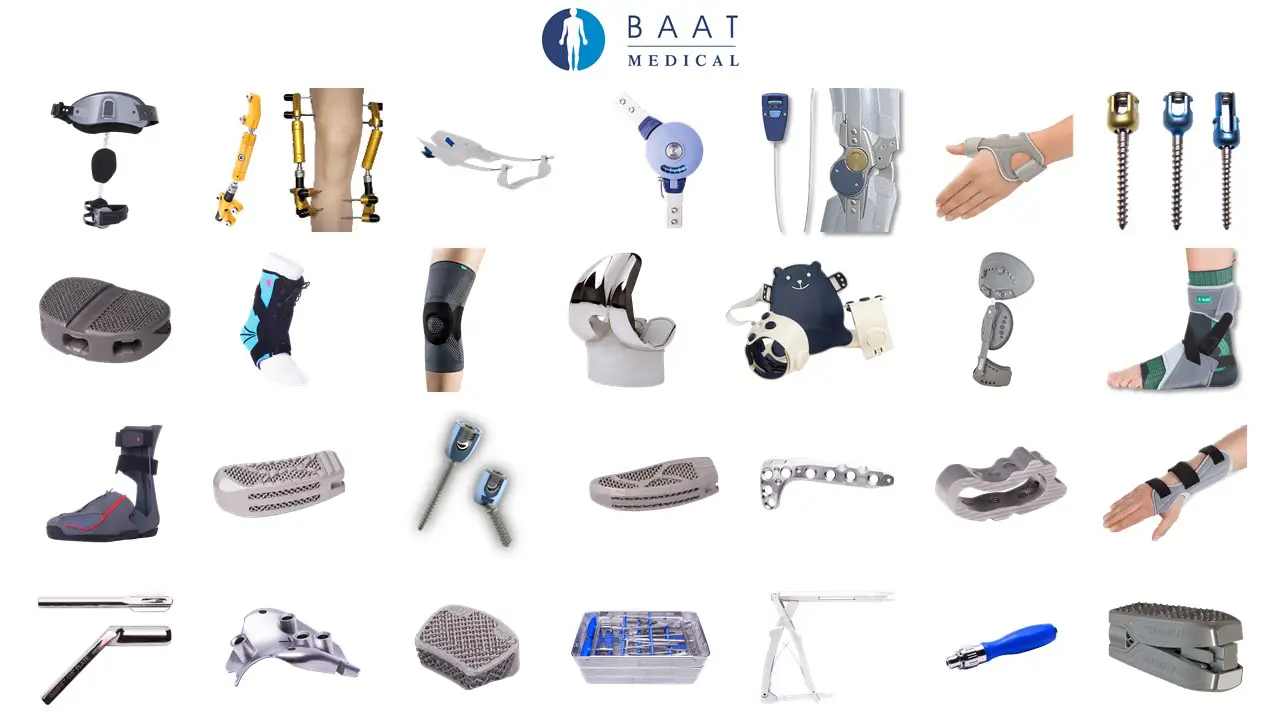
Do you have a great idea and need a partner for the design and manufacture of medical devices? Is your development portfolio bigger than your R&D department’s capacity? BAAT is your partner in expert medical device development from prototype to regulatory approval. BAAT has the experience and know-how to transform your idea into a medical device with manufacturing route, CE/FDA application and operations.
Medical device development is the process used to transform your idea into an approved medical device that is treating patients. BAAT has been the go-to company for medical device development for many customers for over two decades. For us, it is not just about industrial design sketches, medical device prototyping; it is about creating a meaningful impact on the lives of patients.
Along the journey from idea to regulatory approval, you will encounter hurdles. BAAT is successfully guiding customers to overcome these hurdles since 1999. Use this experience to navigate the intricacies of your medical device design and development and get your medical device approved for market introduction.
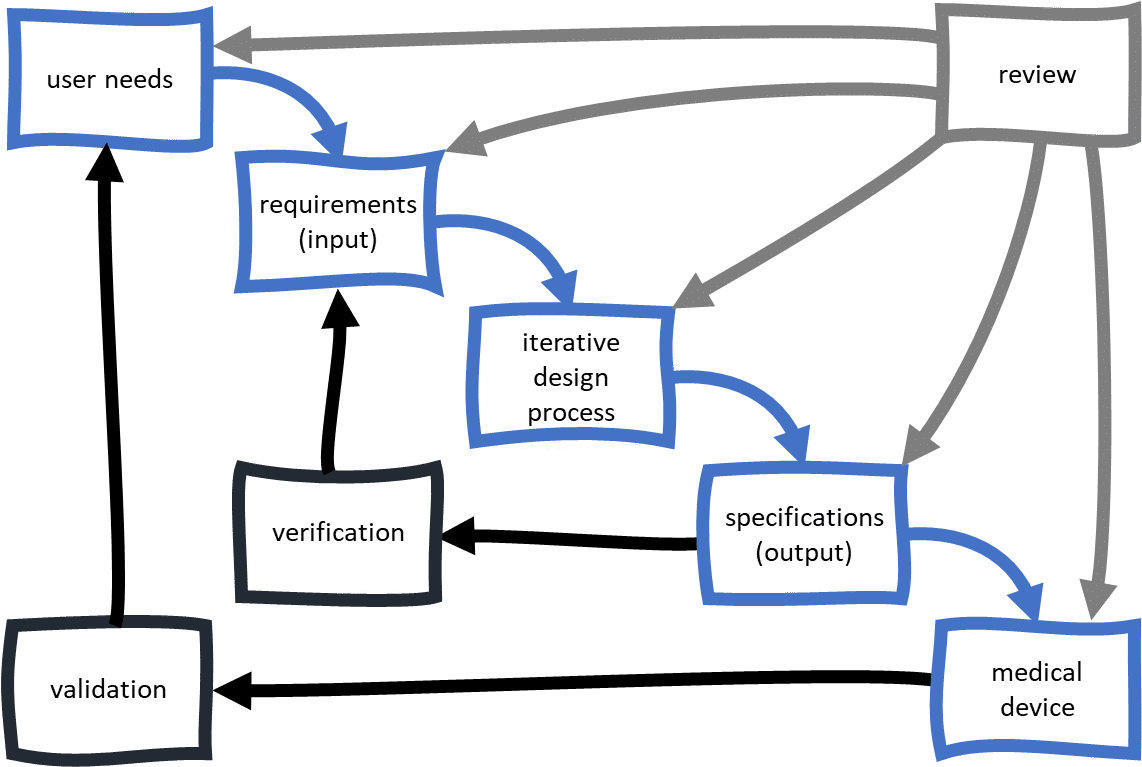
Even the best medical device developers are only as good as the medical device development process they are working under. At least according to the authorities assessing market approval. Strict design controls for the medical device industry are in place, meaning an expert team is not enough to get regulatory approval for a medical device.
They must use a formal medical device development process, with activities focused on design control. The goal of the design control process is the reliable design of medical devices, resulting in products that are safe and effective. For design control, medical device development includes the following topics:
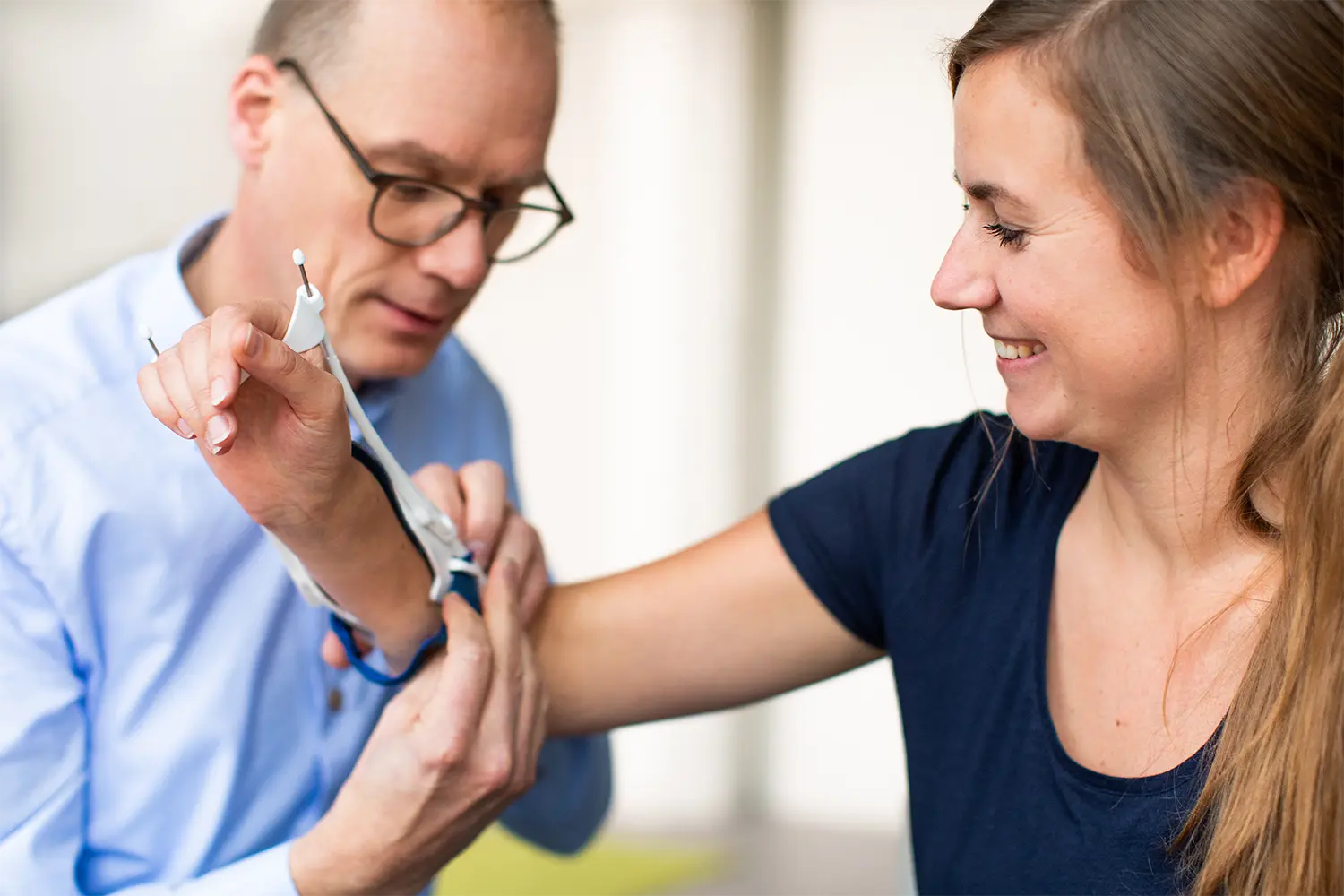
One of the medical device development tools that supports the iterative nature of the process is medical device prototyping. A prototype is a version of your medical device that allows you to review and investigate the design. The question you’re answering defines what medical device prototyping is needed and how much effort medical device prototype development requires.
In medical device prototyping it is important to always use the simplest version of the product with which the most can be learned. During the early stages of the medical device development process, you might learn more from a sketch that allows users to envision the product than from a physical prototype for mechanical evaluation. However, when nearing the finale of your medical device development timelines, the more closely your medical device prototype resembles the final product, the more you learn about it.
Whatever stage of the medical device design process, BAAT understands what questions need answering, and how to use medical prototype development to answer them effectively.
Medical device product development is an iterative design process. That means there are regular design reviews and based on the findings the design is improved. Ultimately, the information created during medical device development forms the design history file. Agencies granting regulatory approval use this file to confirm that your medical device was developed with a design control process.
This design control process for medical devices is specified in a quality management system. Our ISO 13485 quality system concerns topics directly related to medical device design control (e.g. verification and validation, risk management, usability), but also addresses many high-level topics such as competence tracking and training, management of standards, control of documents. When partnering with BAAT for medical product development, you will not only benefit from the knowledge and experience of our 25+ team of experts of various fields, but also our underlying quality system.
BAAT has extensive experience in medical device development for a range of customers. For us, creating the design is just the start. As your full-service MedTech product development company, we are partners for the complete journey and are only satisfied when your medical device is successfully treating patients.