Harnessing Orthosis Development, Innovation, and Product Improvement
Often, we hear that an orthosis is fully developed. There are no significant innovation and the improvements that are made only concern the addition of different sizes, different colors or the use of other materials. However, is this really the case? When is a product fully developed and how can you recognize this?
TRIZ S-Curve
To visualize and analyze the evolution of products, such as orthoses, the TRIZ S-Curve can be used [1] (Figure 1). Each S-Curve shows the evolution of product improvement that consists of three phases: development, introduction and maturity. When connecting multiple S-Curves, one can see the difference between product improvement and innovation (Figure 1).
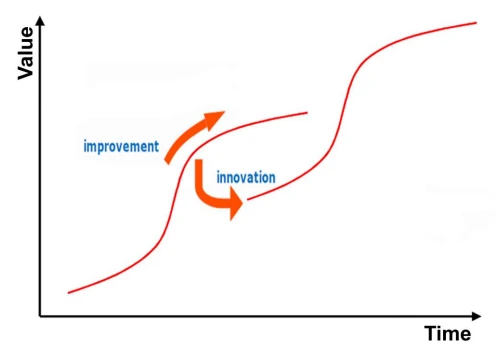
Figure 1 – S-Curve of the TRIZ model [1] showing the evolution of two products towards improvement and innovation. At some point device improvement is no longer profitable and innovation is needed. To make the transition from improvement to innovation, courage is needed as the first version of an innovated device usually underperforms the matured device.
An example: ankle foot orthoses
When focusing on ankle foot orthoses (AFOs), first and second generation AFOs can be identified, indicated by the first and second S-Curve, respectively (Figure 2). First generation AFOs are generally mechanic and provide static support which means that the amount of ankle stiffness is determined before commencing in an activity such as walking. This stiffness may be different into dorsiflexion when compared to plantarflexion direction. Second generation AFOs provide dynamic support which means that the amount of stiffness can vary during an activity. One example is the Unpowered exoskeleton which consists of a clutch to vary support during walking (most right AFO in Figure 2).
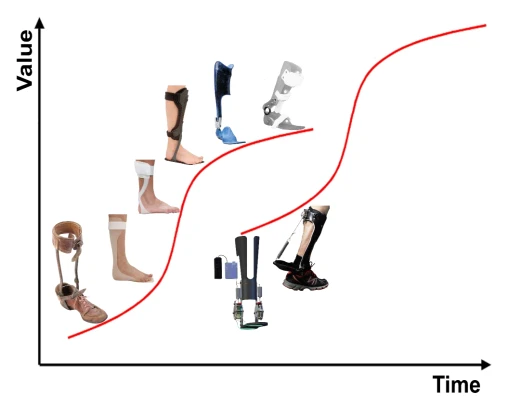
Figure 2 – Development of ankle foot orthoses from left to right: First generation, heavy leather and steel AFOs (C1200) were replaced by lighter plastic AFOs (Posterior Leaf Spring (PLS) & Foot Drop Brace). Variations in trim line resulted in stiffer (PLS) and more flexible AFOs (Foot Drop Brace). The development of new materials such as carbon enabled the production of both lightweight and energy return AFOs (Ground Reaction Force orthosis). A better understanding of patients’ specific ankle stiffness needed into dorsiflexion (DF) and plantarflexion (PF) direction resulted in the development of the Neuro swing and the Orthosis with oil damper [2]. Second generation AFOs can vary the patients’ specific (DF/PF) stiffness during an activity (Adjust [3] & Unpowered exoskeleton [4]).
Conclusion
When to start an innovation is not an exact science but a correct timing is important as it will not be profitable when you make the transition too soon, nor when you make it too late. It can help the decision-making process to evaluate in which phase your medical device currently is. This approach requires a different viewpoint which allows you to get a clear idea on what your focus should be: cost reduction, function improvement, or disruptive innovation.
Example products
At BAAT Medical, we were able to realize some interesting examples of both improved and innovated medical products: