Medical Device Development and Clinical Evaluation Introductionary Whitepaper to Clinical Trials and Investigation BAAT Medical
In the European Union (EU), in general, medical devices must undergo a conformity assessment by a notified body. This assessment should ensure that the device is safe and performs as intended. Performance means that the device meets its intended purpose and that the benefits of using the device outweigh the potential risks associated with the device.
For implants and higher risk (Class III) devices, an important part of the conformity assessment is the evaluation of the results of a pre-market clinical investigation performed with the device.
What is a clinical investigation?
Clinical investigation is the term for a clinical trial using medical devices. A clinical investigation means any systematic investigation involving human subjects, undertaken to assess the clinical performance, safety and effectiveness of a medical device. A clinical investigation is interventional, meaning it evaluates the effects of a therapeutic intervention, which for conformity assessment usually concerns the application of the (new) medical device. Clinical investigations therefore may help in evaluation of medical innovations as well.
Clinical investigations thus result in patient-specific data coming from direct use of the device in or on patients. Note that this excludes studies for which material of human origin is obtained through a third party and for which an investigator has had no direct interaction with the patient, such as cadaver studies or histological analyses.
A clinical investigation should follow a systematic and scientific approach and should be planned, designed, and conducted in accordance with the MDR, of which specifically Chapter VI and Annex XV of the regulation are of interest. Furthermore, to protect patients involved in the study and to produce reliable scientific results, clinical investigations should also comply to ISO 14155.
Following a systematic and scientific approach should be taken very seriously, not only during the set-up and organization of the investigation, but also in the conduct and reporting stage. In case the clinical investigation, or part of it, is not performed according to the rules laid out in the relevant sections of the MDR, a notified body may reject the results as part of the conformity assessment, leaving a gap in the clinical evidence which may hinder market entry.
Regulatory affairs & investigation goals
Before setting up a clinical investigation it is very important to have a clear goal in mind regarding the purpose of the investigation and what regulatory routes to take. Figure 1 shows an overview of different pathways to follow, depending on the status of the device that is subject of the investigation and the investigation goal.
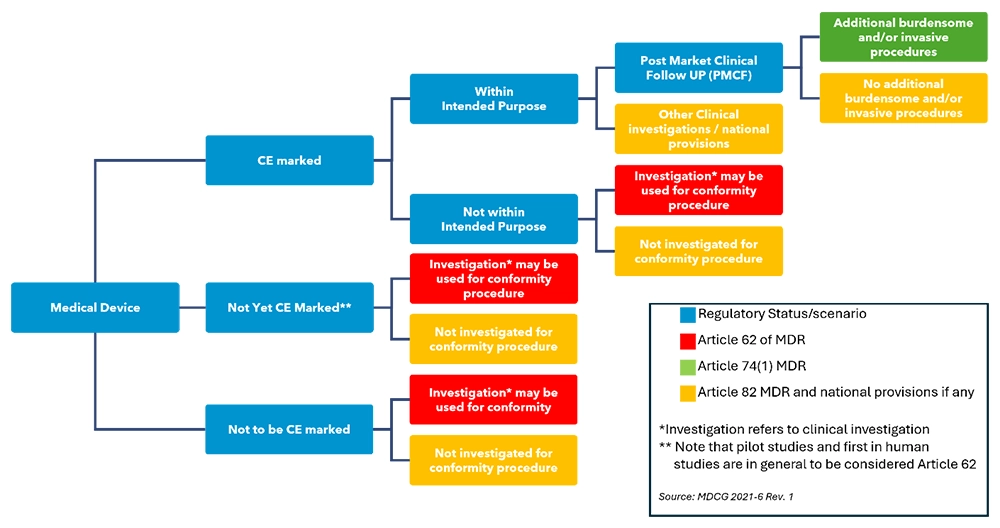
Clinical investigation under the MDR – regulatory pathway, source from MDCG 2021-6, Annex I
In most cases the ultimate goal of conducting a clinical investigation would be to obtain CE mark to enable market entry and sales in countries where the CE mark is accepted for market approval. In that case the clinical investigation should result in a collection of clinical data on use of the (investigational) device within its intended purpose. This data will then be used as input for the conformity assessment by the notified body.
A clinical investigation may also be required as a condition under which a notified body has given a CE mark. Then it becomes part of the post-market strategy to obtain further insight into the safety, performance and benefits of the device. Goal is then to address specific pre-defined safety and performance related issues for which additional data is needed, also within its intended purpose.
A third possible goal for performing clinical investigations, with devices already bearing the CE-mark, can be to collect clinical data on the use of a device outside of its intended purpose, for example to assess a broader scope of use of the device.
Roles and responsibilities
The planning and execution of a clinical investigation is a complex process and involves many stakeholders. Stakeholders that are involved in a clinical investigation may include:
- The governmental Competent Authority (CA)
- The Ethics Committee (EC)
- The Principal Investigator (PI)
- The sponsor
- The (legal) manufacturer (LM)
- The involved hospitals
- The CRO
- The patient
- The insurance company
Within a clinical investigation, each of these potential stakeholders has a specific role, and needs specific information for execution of this role. Aligning these roles, the different accompanying interests and the necessary information needed for each role is complex and takes time. Especially for pre-market clinical investigations, it easily takes more than a year between start and receiving actual study approval. In a next article the role of each stakeholder will be discussed in more detail.
Get support for your clinical investigation or trial
BAAT Medical can help you to understand and align the various roles and stakeholders and their needs in order to get a clinical investigation approved within a reasonable timeframe. We even can play one or more of these roles to support your organization and take care that you deliver the right documentation, at the right time, with the right content.